Theme: Research and development of therapeutic agents and vaccines for COVID-19 using pharmaceutical biotechnology
Euro Pharmaceutical Biotechnology 2020
The Organizing Committee Members is esteemed to invite you to join the 25th International Congress on Pharmaceutical Biotechnology on June 24-25, 2020 which includes prompt keynote presentations, Oral talks, Poster presentations, Workshops and Exhibitions.
Join your peers around the world focused on learning about Bio pharmaceutics, Biotechnology, Biologics and Biosimilars related advances, which is your single best opportunity to reach the largest assemblage of participants from the Biotechnology community, Biopharma community, distribute information, meet with current and potential professionals, make a splash with a new research works, and receive name recognition at this 2-day event. World-renowned speakers, the most recent research, advances, and the newest updates in Biologics and Biosimilars are hallmarks of this conference. The Organizing Committee Members of EURO PHARMACEUTICAL BIOTECHNOLOGY 2020 also invite Young Researchers to submit abstracts reporting their latest scientific findings for Poster sessions.
Theme: Research & Development on Therapeutic Agents and Vaccines for COVID-19 using Pharmaceutical Biotechnology
Why to attend?
- Access to All Sessions Online
- E-Handbook & E-Conference Kit
- E-Certificate of Presentation
- Online publication of Abstract and Biography in our website which has 25 Million visitors
- Online publication of Abstract and Biography in respective conference webpage which has 25,000 online unique subject experts visitors
Target Audience:
- Biopharma, Biotech, Pharma Industry CEO, Directors, R&D Researchers
- Business Entrepreneurs, Training Institutes, Software developing companies,
- Medical Devices Manufacturing Companies, CRO
- Data Management, Clinical Trial Conducting Organizations and Companies
- Students, Scientists, Researchers, and Faculty of Pharmaceutical Sciences
- Universities, Medical Colleges, Researchers from Pharmaceutical background
The EURO PHARMACEUTICAL BIOTECHNOLOGY online event is scheduled on June 24-25, 2020
Track-1: Biopharmaceuticals
A Biopharmaceutical is also called as a Biological pharmaceutical medication item fabricated from organic sources through extraction. The advancement of a pharmaceutical medication normally takes a normal of 10-15 years. Such a large amount of exertion, time and cash, it is exceptionally urgent that the correct advancements and materials are received in the examination, improvement and assembling of the pharmaceutical medications, whole process incorporates pre-disclosure, pre-clinical trials, showcase dispatch to post promoting observing. The wellsprings of medication items incorporate peptides and proteins, including monoclonal antibodies and neutralizer pieces.
Track-2: Pharmaceutical Biotechnology
Pharmaceutical Biotechnology is the broad area of biology involving living systems and organisms to develop or make products, or "any technological application that uses biological systems, living organisms, or derivatives thereof, to make or modify products or processes for specific use". Depending on the tools and applications, it often overlaps with the related fields of molecular biology, bio-engineering, biomedical engineering, bio manufacturing, molecular engineering, etc. Biotechnology has expanded to include new and diverse sciences such as genomics, recombinant gene techniques, applied immunology, and development of pharmaceutical therapies and diagnostic tests.
- Biomedical Data Engineering
- Bioinformatics Engineering
- Industrial Pharma Biotechnology
- Genetic Engineering and Tissue Engineering
Track-3: Biopharmaceutical Informatics
Biopharmaceutical informatics endeavors to use information technology, sequence-and structure-based bioinformatics analyses, molecular modelling and simulations, and statistical data analyze towards biologic drug development. Development of databases containing the data on the biophysical stability, safety along with molecular sequence.
- Applications of Computation in Biologic Drug Development
- Physics-based molecular modelling
- Protein sequence-structural contexts and degradation reaction mechanisms
- Pre-clinical immunogenicity risk assessment of bio therapeutics
Track-4: Biotechnology and its Applications
Biotechnology it is a piece of science that arrangements with utilization of living frameworks, by applying any innovative applications. That utilizations natural frameworks, living life forms, or subsidiaries thereof, to make items or procedures for particular utilize. Utilizations of biotechnology for the most part centered on Agro concoction agribusiness, Organic farming, and yield based horticulture. Uses of Biotechnology in Food Processing are Environmental Biotechnology and Biotechnology in Medicine, Industrial Biotechnology.
- To develop useful and beneficial plants
- Process of traditional plant breeding
- Improvement of agricultural production
- Applications of environmental management
- Applications of agriculture
Track-5: Biopharmaceutical Pharmacovigilance
The field of Pharmacovigilance is growing rapidly and its development is making tremendous impacts in medical sciences and pharmaceuticals. Euro Biopharma 2017 emphasizes on how the importance and significance can be gauged by the fact that it has made huge advancements over the course of time and is continuing to influence various sectors.
- Drug Safety
- Adverse Drug Reaction
- Pharmacovigilance and Risk Management
- Pharmacovigilance Significance and Scope
Track-6: Innovations in Biotech Manufacturing
Biotechnology is the utilization of living frameworks and life forms to make items, by any innovative applications to change items or procedures for particular utilize. Biotechnology has applications in four noteworthy modern regions, including Health mind, Crop generation and horticulture, Industrial employments of harvests and different items and Environmental employments. Biotechnology has likewise prompted to the advancement of anti-infection agents. Yield of a generation procedure are an ideal opportunity to aggregate a coveted measure of biomass, the procedure term, and the particular profitability. By contrasting greatest cell densities and particular development rates of different expression frameworks, and extensively depicted logical methodologies and procedures to enhance have cell lines. Other than the quantitative assessment of current frameworks, the quality-deciding properties of a host cell line.
- Molecular biotechnology
- Techniques of biotech Pharma
- Engineering machineries
- New biotech Pharma drug design
Track-7: Biotechnological Products
Biotechnology is frequently used to allude to hereditary designing innovation of the 21st century. However, the term is utilized for some methods for changing natural life forms for the necessities of humankind. It began with changes of local plants into enhanced sustenance edits through fake determination and hybridization. Bioengineering is the science where upon all biotechnological applications are based. With the advancement of new methodologies and present day procedures, conventional biotechnology ventures are additionally securing new skylines empowering them to enhance the nature of their items and increment the profitability of their frameworks.
- Protein Interactions
- Herbal Drug Interactions
- Nanoparticle drugs
- Drug Elimination and Clearance
- Pharmacogenomics
- Bioinformatics
Track-8: Clinical Trials on Biopharmaceutical Products
In the fields of prescription, biotechnology and pharmacology, sedate revelation is the procedure by which new candidate medications are found. Generally, medications were found through recognizing the dynamic fixing from customary cures or by fortunate revelation. Later compound libraries of manufactured little particles, characteristic items or extracts were screened in place cells or entire life forms to recognize substances that have an alluring remedial impact in a procedure known as traditional pharmacology. Since sequencing of the human genome which permitted fast cloning and amalgamation of vast amounts of cleaned proteins, it has ended up basic practice to utilize high throughput screening of expansive mixes libraries against confined organic targets which are speculated to be illness altering in a procedure known as turnaround pharmacology.
Case Studies at Different Trial Phases
- Pre -Clinical Studies
- Regulatory Update
- Clinical Design
- Upcoming Challenges in Clinical Trails
- Pyrogen Testing
Track-9: Chemical Biotechnology
Biotechnology is defined as the application of the life sciences to chemical synthesis. This is a study of direct production of specific chemicals via fermentation, such as lactic acid and citric acid; Now-a-days chemical biotechnology is increasing biotechnology’s contribution to the industries. Chemical biotechnology mainly includes in both chemical engineering and scientific principles. Chemical engineer helps to design, production of microorganisms and enzymes to synthesize new drugs. Huge number of industries that depend on the synthesis and processing of chemicals and materials. This mainly deals with the oil industries, pharmaceutical industries. Chemical engineers enjoy increasing opportunities.
- Chemical disinfectant testing
- Cleaning studies
- Particulate testing of chemicals
- Sampling and analysis of solvents
- Biofuels in chemical industry
- Biotechnology in chemical industry
- Controlled drug delivery
- Mechanisms of Action and Toxicity
- Factors Affecting Drug Metabolism
Track-10: Biopharmaceutical Engineering
Pharmaceutical building is a piece of pharmaceutical science. The touchy advancement in the pharmaceutical business turned into the main thrust for our new Biopharmaceutical Engineering Program. The branch of biopharmaceuticals is quickly forming into upstream medication definitions and downstream plan handle, by utilizing biopharmaceutical designing standards.
- Biomaterials
- Tissue biomaterials
- Pharmaceutical engineering
- Drug Elimination and Clearance
- Pharmacogenomics
- Pronuclear Micro Injection Method
- Bioinformatics
Track-11: Nanoparticles in Biopharmaceuticals
Nanoparticles with the end goal of medication conveyance are characterized as submicron, which are small colloidal particles. Nanoparticles are strong colloidal lattice like particles which are made of polymers or lipids. For the most part directed by the intravenous course like parenteral they have been created for the focused on conveyance of helpful or imaging specialists. By methods for focused medication conveyance framework will be accomplished rapidly, to enhance the pharmacokinetic and pharmacodynamics movement of the drug.
- Biochemical tools for investigating
- Engineering applied to the biosciences
- Biosynthetic products structure modification
- Formulation of biotech products
- Screening and synthesis of Nano drug design
- Biodegradable microspheres
Track-12: Regulatory Affairs in Biopharmaceuticals
Regulatory Science is the science of advanced standards equipment, and paths to assess the safety, Drug toxicity and quality, potency of all FDA-regulated products. An access to lengthen the programs in regulatory science that leverages what has been well-educated in the development of training programs for translational scientists, and this model for regulatory science program development is being refined and adopted by all of the institutions that are part of the CTSA network. The target audience for such a program is broad, noted that it is necessary to break out of the mind-set that regulatory science resides totally with FDA and that the field's purpose is to create a workforce that will function within the FDA. Critical needs for a regulatory science training program understand research and scientific methodology, toxicology, therapeutics, and pharmacology that underpin the regulatory process.
- Biopharma Regulatory challenges
- Regulatory Approvals for Biopharma drug products
- Overview of drug, biologic and device regulatory pathways
- Role of Regulatory Authorities
- BPCI Act
- Hatch-Waxman Act
Track-13: Biopharmaceutical Drug Design and Development
Drug design is an inventive process of finding new medications of a biological target which frequently but not necessarily relies on computer modelling techniques use of high throughput screening techniques to analyze new compounds, both by synthetic and natural, as novel drugs. Regrettably, this approach has yielded very little achievement in the field of anti-infective drug discovery. The identification of both molecular targets that are essential for the survival of the pathogen, and compounds that are active on intact cells, is a challenging task.
- Pharmacogenomics Drugs
- Biological products Used in Drug Discovery
- Human Genomic Project
- Cell based Therapies
Track-14: Pharmaceutical Analysis
Pharmaceutical Analysis is about studying and knowing about the uses of instruments and methods which used to separate, identify, and quantify matter. In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analyses. Qualitative analysis identifies analyses, while quantitative analysis determines the numerical amount or concentration. It consists of classical, wet chemical methods and modern, instrumental methods. Classical qualitative methods use separations such as precipitation, extraction, and distillation. Identification may be based on differences in colour, odour, melting point, boiling point, radioactivity or reactivity.
Analytical chemistry is also focused on improvements in experimental design, chemo metrics, and the creation of new measurement tools. Analytical chemistry has broad applications to forensics, medicine, science and engineering.
- Novel Approaches to Analytical and Bio analytical Methods
- Bio analytical Techniques
- Chromatography Techniques
- Spectroscopic Techniques
- Nuclear Magnetic Resonance
- Mass Spectroscopy
- Regulatory Issues and Biosafety Challenges in Bio analysis
- Applications of Analytical and Bio analytical Methods
Track-15: Biotechnology in Health Care
Biotechnology is the utilization of living frameworks and creatures to make items, by any innovative applications to make or adjust items or procedures for particular utilize. Science and innovation have in the past assumed an indispensable part in enhancing general wellbeing. Today the commitment of science to enhance general wellbeing and lessen worldwide wellbeing incongruities is more apropos than any other time in recent memory.
- Green Technology
- Biotechnology and Diabetes
- Food and industrial biotechnology
- Biomedical engineering
- Medical biotechnology
- Biotech Pharma quality issues
Track-16: Agriculture Biotechnology
Rural biotechnology is otherwise called “Agritech”. Which including the utilization of logical instruments and systems, including hereditary designing, a surge in innovation brought about an expansion in horticultural biotechnology through the determination of qualities like expanded yield. Agriculturists have controlled plants and creatures through specific reproducing for a huge number of years with a specific end goal to make wanted characteristics. Horticultural Biotechnology has been utilized to enhance the healthful substance of an assortment of products with an end goal to address the issues of an expanding populace.
- Stem cell biotechnology
- Prokaryotic cells in biotech production
- Microbial origin
- Fermentation process
- Pharmacokinetics and pharmacodynamics
Track-17: Formulation of Biotech Products
Biotech medicate formulators have many worries to juggle in their work, starting with the physicochemical qualities of a dynamic particle and including the dependability, cost, and accessibility of logical techniques utilized as a part of detailing work .The results of biotechnology are proteins and peptides that are generally flimsy atoms contrasted with most natural pharmaceuticals. At last the protein is traded into its last arrangement dose frame where long haul strength is accomplished. These incorporate rankles, bottles, vials, ampules, syringes.
- Culture manipulation of biological products
- Regulatory issues and drug approval
- Biotech drug design
- Evaluation studies
- Chromatography techniques
Track-18: Biotech Companies and Market Analysis
The biotechnology division is profoundly inventive. Which has solid development direction, with its colossal development potential, will keep on playing a noteworthy part as an imaginative assembling centre. The division is a standout amongst the most noteworthy parts in upgrading worldwide profile and additionally adding to the development of the economy. Biopharma is the biggest part contributing around 64 for every penny of the aggregate income took after by bio services.
- Modern biotech market analysis
- Economic considerations in medical
- Products supply statistics
- Market research reports & industry analysis
- Companion technologies and markets
- Regulatory Aspects
Market analysis
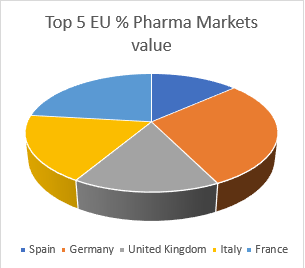
The global pharmaceuticals market was worth $934.8 billion in 2017 and will reach $1170 billion in 2021, growing at 5.8%, according to a recent pharma market research report by The Business Research Company. The Pharmaceutical Market in EU has a value of over 183 Million €, positioning Europe as the second biggest pharmaceutical market in the world, with Germany, France, Italy, United Kingdom and Spain as top 5 countries. The largest market is Germany with around 48 Million € which accounts for 24% of the market.
Europe accounts for 22% of world pharmaceutical sales, during the period 2012-2017 and 18% of sales of new drugs were on the European market. The total volume of medicinal products consumed globally is set to grow about 3% annually through 2021 and estimations point to a compound annual growth rate of 4 percent by 2022.The European injectable drug delivery market is expected to reach $207.3 Billion by 2020 from $114.7 Billion in 2015, growing at a CAGR of 12.6% from 2015 to 2020. Factors such as rising prevalence of chronic diseases, growth of the biologics market, demand of self-injection devices, and technological advancements are driving the growth of this market. On the other hand, alternative delivery methods such as oral and transdermal, safety concerns, and blood-borne infections are hindering the growth of this market. The North American injectable drug delivery technologies market was valued at $9.3 billion in 2012; it is expected to reach $16.6 billion by 2017 at a CAGR of 12.3% from 2012 to 2017. The pharmaceutical market in Asia is set to rise from around $70 billion in 2016 to $72 billion by 2021, representing a low compound annual growth rate of 0.3%, according to Global Data, a recognized leader in providing business information and analytics. The global bioinformatics market in 2018 is $7.5 billion to $20.0 billion by 2023, with a compound annual growth rate (CAGR) of 21.7% during the period of 2018-2023.

The pharmaceutical market in Asia is set to rise from around $70 billion in 2016 to $72 billion by 2021, representing a low compound annual growth rate of 0.3%, according to Global Data, a recognized leader in providing business information and analytics. The global biotechnology market is expected to reach USD 727.1 billion by 2025, according to a new report by Grand View Research, Inc.
The Netherlands is a leader in the life sciences and biotech industry, ranked 4 worldwide for medical technology patent applications and ranked 9 worldwide for biotechnology patent applications. Many leading multinational pharmaceutical businesses have established operations in the Netherlands, including MSD (also known as Merck & Co.), Janssen pharmaceutical companies (a division of Johnson & Johnson), and Amgen. Their operations cover everything from R&D and production to logistics and marketing.
Pharmaceutical Biotechnology 2019
Thanks for attending Biotechnology 2019!!
We gratefully thank all our wonderful Speakers, Conference Attendees, Students, Media Partners, Associations and Exhibitors for making The 24th International meet on Pharmaceutical Biotechnology the best ever!
Biotechnology 2019 is distinguished with the attendance of Editorial Board Members of supported Journals, Scientists, Young and Brilliant Researchers, Business Delegates and Talented Student communities representing more than 25 countries who made this conference rewarding and fecund.
Our 24th International meet on Pharmaceutical Biotechnology was based on the theme “Providing a global platform to explore and enhance the future of medicine and pharmaceutical biotechnology” The meeting covered various sessions, in which the discussions included the scientific tracks:
- Pharmaceutical Biotechnology
- Medical Biotechnology
- Medical Biotechnology
- Stem Cell Biotechnology
- Cancer Biotechnology
- Biopharmaceutical Formulations
- Pharmacokinetics and Pharmacodynamics
- Nano biotechnology
- Agricultural Biotechnology
- Bio Informatics
- Recombinant DNA technology
- White Biotechnology
- Marine Biotechnology
- Environmental Biotechnology
- Biotechnology on Food processing
- Clinical Research/Clinical Trails
The Keynote presentations were given by:
Sandra Acosta | Orion Scientific Group, USA
Steven Soldin | National Institutes of Health, USA
Marie-Lise | CHU UCL Namur ( Belgium )
Conference Series LLC Ltd wishes to acknowledge with its deep sincere gratitude to all the supporters from the Editorial Board Members of our Open Access Journals, Keynote speakers, Valuable speakers, Poster presenters, students, delegates and special thanks to the Media Partners for their promotion to make this event a huge success.
We once again thank you all for the enormous exquisite response. This inspires us to continue organizing events and conferences for furthering the Biotechnology. Conference Series LLC ltd therefore, is glad to announce its “25th International Congress on Pharmaceutical Biotechnology” slated on June 24-25, 2020 Amsterdam | Netherlands
Mark your calendars for the upcoming meeting; we are hoping to see you soon!!!!
For More details visit: https://biotechnology.pharmaceuticalconferences.com/
Organizing Committee
Biotechnology 2020
Conference Highlights
- Biopharmaceuticals
- Pharmaceutical Biotechnology
- Biopharmaceutical Informatics
- Biotechnology and its Applications
- Biopharmaceutical Pharmacovigilance
- Innovations in Biotech Manufacturing
- Biotechnological Products
- Clinical Trials on Biopharmaceutical Products
- Chemical Biotechnology
- Biopharmaceutical Engineering
- Nanoparticles in Biopharmaceuticals
- Regulatory Affairs in Biopharmaceuticals
- Biopharmaceutical Drug Design and Development
- Pharmaceutical Analysis
- Biotechnology in Health Care
- Agriculture Biotechnology
- Formulation of Biotech Products
- Biotech Companies and Market Analysis
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | June 24-25, 2020 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Biomedical and Pharmaceutical Sciences
- Der Pharmacia Lettre
- Research & Reviews in Pharmacy and Pharmaceutical Sciences
Abstracts will be provided with Digital Object Identifier by





